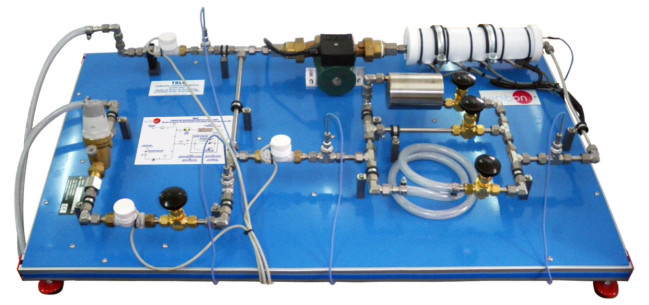
TBCF Kalorimetrische Pumpe
INNOVATIVE SYSTEME
Bomb Calorimeter, "TBCF", is a classic device used to determine the heating or calorific value of solid and liquid fuel samples at constant volume.
Erweiterungen
Laboratorien
ÄHNLICHE NEUIGKEITEN
ALLGEMEINE BESCHREIBUNG
Bomb Calorimeter, "TBCF", comprises the calorimeter, a calorimeter vessel, an outer double walled water jacket, control unit to switch on/off the stirrer and the ignition device, an accurate thermometer and charging unit with pressure gauges to facilitate the charging of the calorimeter with oxygen.
The calorimeter vessel and outer jacket wall are manufactured in stainless steel.
The bomb calorimeter is a container made of stainless steel that can support high pressures. It is sealed by a screw top. The bomb is charged with gas (oxygen) through the filling valve. This bomb is introduced inside a calorimeter vessel made of stainless steel that is filled with water, and at the same time it is introduced inside a double walled water jacket.
The rod of the calorimeter supports a metallic crucible. The bomb calorimeter, which contains the fuel sample to be burned, is hermetic to the gas by closing the filling valve and its cover. Combustion is started through a thin wire that is red hot-heated up momentarily due to the passing of an electrical current that flows through an isolated terminal and the rod, which is electrically connected to the cover.
The water in the calorimeter vessel is agitated automatically with a stirrer driven by a small motor. The top of the double walled jacket is closed with a cover that has some orifices. An accurate thermometer to measure the temperature of the calorimeter vessel passes throughone of these orifices. Other orifices are used to fasten the jacket to the cover. Also, one of these holes is used to insert the wire that supplies the electric current to the rod.
The unit includes a control unit that switches on/off the stirrer and the ignition device through the heating up of the thin wire and a load unit with pressure gauges to make the filling with oxygen of the calorimeter easier.
ÜBUNGEN UND GEFÜHRTE PRAKTIKEN
GEFÜHRTE PRAKTISCHE ÜBUNGEN IM HANDBUCH ENTHALTEN
- Obtaining the calorific value of fuel.
- Performing experiments to measure heats of combustion.
- Calculating the heats of combustion from experimental results.
- Calculating internal energies of combustion from bomb calorimeter experiments.
- Calculating enthalpies of combustion from bomb calorimeter experiments.
ERGÄNZENDE AUSRÜSTUNG
Gerät für praktische Übungen zur thermischen Ausdehnung
Gerät für Gasexpansionsprozesse eines idealen Gases, computergesteuert (PC)
Drossel- und Trennkalorimeter, computergesteuert (PC)
Marcet-Kesselgerät, computergesteuert (PC)
Gerät zur Temperaturmessung, computergesteuert (PC)
Gerät zur Temperaturmessung
Trainingsgerät für Temperaturmessungen
Gerät für die Untersuchung der Gasgesetze (Boyle und Gay-Lussac), computergesteuert (PC)
Gerät für Recycling-Schleifen, computergesteuert (PC)
Gerät für Recycling-Schleifen
Gerät für Sättigungsdruck, computergesteuert (PC)
QUALITÄT

KUNDENDIENST

 Cookie-Präferenzen
Cookie-Präferenzen