EEC Korrosionstestgerät.
INNOVATIVE SYSTEME
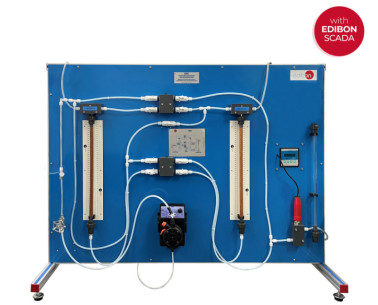
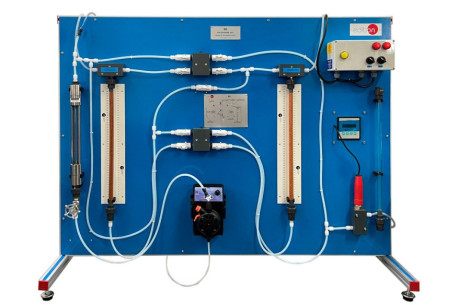
The Corrosion Study Unit, "EEC", allows the corrosion simultaneous study up to eight corrosion cells. This unit allows to study several conditions that influence in the corrosion processes.
Erweiterungen
Laboratorien
ÄHNLICHE NEUIGKEITEN
ALLGEMEINE BESCHREIBUNG
The Corrosion Study Unit, "EEC", allows the corrosion simultaneous study up to eight corrosion cells. This unit allows to study several conditions that influence in the corrosion processes.
Each sample for the test is put in such away that unwanted secondary effects are eliminated.
The cells included a cover to adapt two test sheets, a reference electrode and a gas diffuser tube.
A salt bridge is included for the study and production of electrochemical cells.
It is possible connect the different samples to a power supply to study the principle of sacrificial anodes.
The ambient air is introduced into the electrolyte solution with an air pump. An air flowmeter is situated at the pump outlet to measure the air flow. There are several air control valves to adjust the air flow rate for each corrosion cell. It is also possible to feed other gases into the electrolyte solution using a gas flowmeter and several gas control valves.
The service panel includes a miliammeter and a milivoltmeter to measure the intensity and the voltage between the electrodes, a voltmeter/ammeter selector, a cell selector and several connecting terminals in the test sheets.
The unit includes a pH-meter to study and compare the influence of the electrolyte solution on the corrosion processes.
The corrosion rate of a certain sample can be measured qualitatively by "visual observation", or quantitatively though "immersion test by initial and final weighing".
ÜBUNGEN UND GEFÜHRTE PRAKTIKEN
GEFÜHRTE PRAKTISCHE ÜBUNGEN IM HANDBUCH ENTHALTEN
- Galvanic potentials.
- Study of galvanic couples.
- Passivation of iron.
- Influence of pH.
- Aluminum anodizing process.
- Cathodic protection.
- Galvanic corrosion + oxidation.
- Daniell cell construction to obtain standard potentials.
MEHR PRAKTISCHE ÜBUNGEN FÜR DAS GERÄT
- Influence of the O2 concentration in the corrosion.
- Simultaneous study of corrosion in several cells.
- Electrolytic corrosion.
- Chemical inhibition.
- Prevention of scaling.
- Effect of internal stress.
- Water treatment studies:
- Calcium carbonate stabilization.
- Oxidation of iron and manganese in ground waters.
- Water softening by chemical precipitation.
- Desinfection of waste water with chlorine solutions.
ÄHNLICHE VERFÜGBARE GERÄTE
ERGÄNZENDE AUSRÜSTUNG
Computer Controlled Corrosion Study Unit
Computer Controlled Ion Exchange Unit
Ionenaustauschgerät.
QUALITÄT

KUNDENDIENST

 Cookie-Präferenzen
Cookie-Präferenzen