TBCF Калориметрический насос
ИННОВАЦИОННЫЕ СИСТЕМЫ
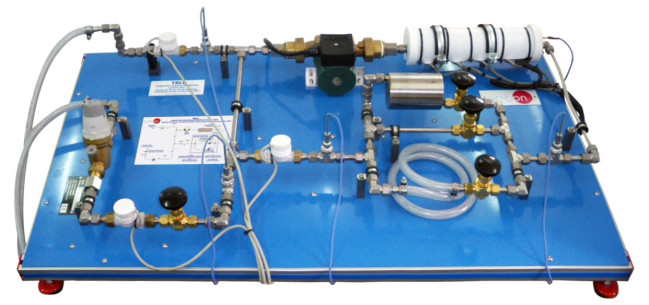
Bomb Calorimeter, "TBCF", is a classic device used to determine the heating or calorific value of solid and liquid fuel samples at constant volume.
ЛАБОРАТОРИИ
НОВОСТИ ПО ТЕМЕ
ОБЩЕЕ ОПИСАНИЕ
Bomb Calorimeter, "TBCF", comprises the calorimeter, a calorimeter vessel, an outer double walled water jacket, control unit to switch on/off the stirrer and the ignition device, an accurate thermometer and charging unit with pressure gauges to facilitate the charging of the calorimeter with oxygen.
The calorimeter vessel and outer jacket wall are manufactured in stainless steel.
The bomb calorimeter is a container made of stainless steel that can support high pressures. It is sealed by a screw top. The bomb is charged with gas (oxygen) through the filling valve. This bomb is introduced inside a calorimeter vessel made of stainless steel that is filled with water, and at the same time it is introduced inside a double walled water jacket.
The rod of the calorimeter supports a metallic crucible. The bomb calorimeter, which contains the fuel sample to be burned, is hermetic to the gas by closing the filling valve and its cover. Combustion is started through a thin wire that is red hot-heated up momentarily due to the passing of an electrical current that flows through an isolated terminal and the rod, which is electrically connected to the cover.
The water in the calorimeter vessel is agitated automatically with a stirrer driven by a small motor. The top of the double walled jacket is closed with a cover that has some orifices. An accurate thermometer to measure the temperature of the calorimeter vessel passes throughone of these orifices. Other orifices are used to fasten the jacket to the cover. Also, one of these holes is used to insert the wire that supplies the electric current to the rod.
The unit includes a control unit that switches on/off the stirrer and the ignition device through the heating up of the thin wire and a load unit with pressure gauges to make the filling with oxygen of the calorimeter easier.
УПРАЖНЕНИЯ И ПРИМЕРЫ С ИНСТРУКЦИЯМИ
РУКОВОДСТВО ПО ПРАКТИЧЕСКИМ УПРАЖНЕНИЯМ ВКЛЮЧЕНО В РУКОВОДСТВО ПОЛЬЗОВАТЕЛЯ
- Obtaining the calorific value of fuel.
- Performing experiments to measure heats of combustion.
- Calculating the heats of combustion from experimental results.
- Calculating internal energies of combustion from bomb calorimeter experiments.
- Calculating enthalpies of combustion from bomb calorimeter experiments.
ДОПОЛНИТЕЛЬНОЕ ОБОРУДОВАНИЕ
Оборудование для практики теплового расширения
Оборудование для процессов расширения идеального газа, управляемое компьютером (ПК)
Термокалибратор с разделением, управляемый с компьютера (ПК)
Оборудование с котлом Марсета, управляемое компьютером (ПК)
Оборудование для измерения температуры, управляемое компьютером (ПК)
Оборудование для измерения температуры
Учебное оборудование для измерения температуры
Оборудование для законов газов (законы Бойля и Гей-Люссака), управляемое компьютером (ПК)
Оборудование для циклов рециркуляции, управляемое компьютером (ПК)
Оборудование для циклов рециркуляции
Оборудование для насыщенного давления, управляемое компьютером (ПК)
КАЧЕСТВО

ПОСЛЕПРОДАЖНОЕ ОБСЛУЖИВАНИЕ

 Настройки cookie
Настройки cookie